Your UM director just told you the team averaged 8.5 days on standard prior auths last quarter. You nodded, made a note, moved on. In six months, that number becomes a regulatory violation.
For years, health plans have complained about prior authorization burdens: opaque decisioning, variable outcomes, slow turnaround, escalating provider frustration. Half-hearted automation efforts and hybrid analog-digital processes made the problem more visible without solving it.
CMS is now codifying expectations in a way that forces every payer to face reality: the way prior authorization has been done cannot survive 2026.
The changes coming from the CMS Interoperability and Prior Authorization Final Rule aren't incremental technical requirements. They're operational inflection points that will expose long-standing design flaws in prior authorization and utilization management. Leaders who wait until enforcement deadlines will find themselves reacting. Those who act now can redesign the system itself.
The Question Leaders Should Be Asking
Most plans are asking: "What do we have to do to comply with CMS by 2026?" That's a tactical question.
The strategic question is: How do we redesign our prior authorization engine so it performs at the speed, transparency, and explainability levels CMS expects without burning clinical resources, inflating costs, or fragmenting operations?
Checking boxes gets you compliant. Redesigning the system gets you competitive.
What Actually Changes in 2026
Beginning January 1, 2026, CMS moves prior authorization from operational best practice to regulatory mandate.
Under the Interoperability and Prior Authorization Final Rule (CMS-0057-F), impacted payers including Medicare Advantage, Medicaid managed care organizations, CHIP, and certain Qualified Health Plans must comply with several non-negotiable requirements1:
72-hour turnaround for expedited prior authorization requests
Seven calendar days for standard requests
Specific, actionable denial reasons included with every adverse determination
Public reporting of prior authorization metrics including approval rates, denial rates, and average processing times beginning March 31, 2026.2
FHIR-based APIs to support electronic prior authorization workflows and expanded data access
Here's what this means in practice:
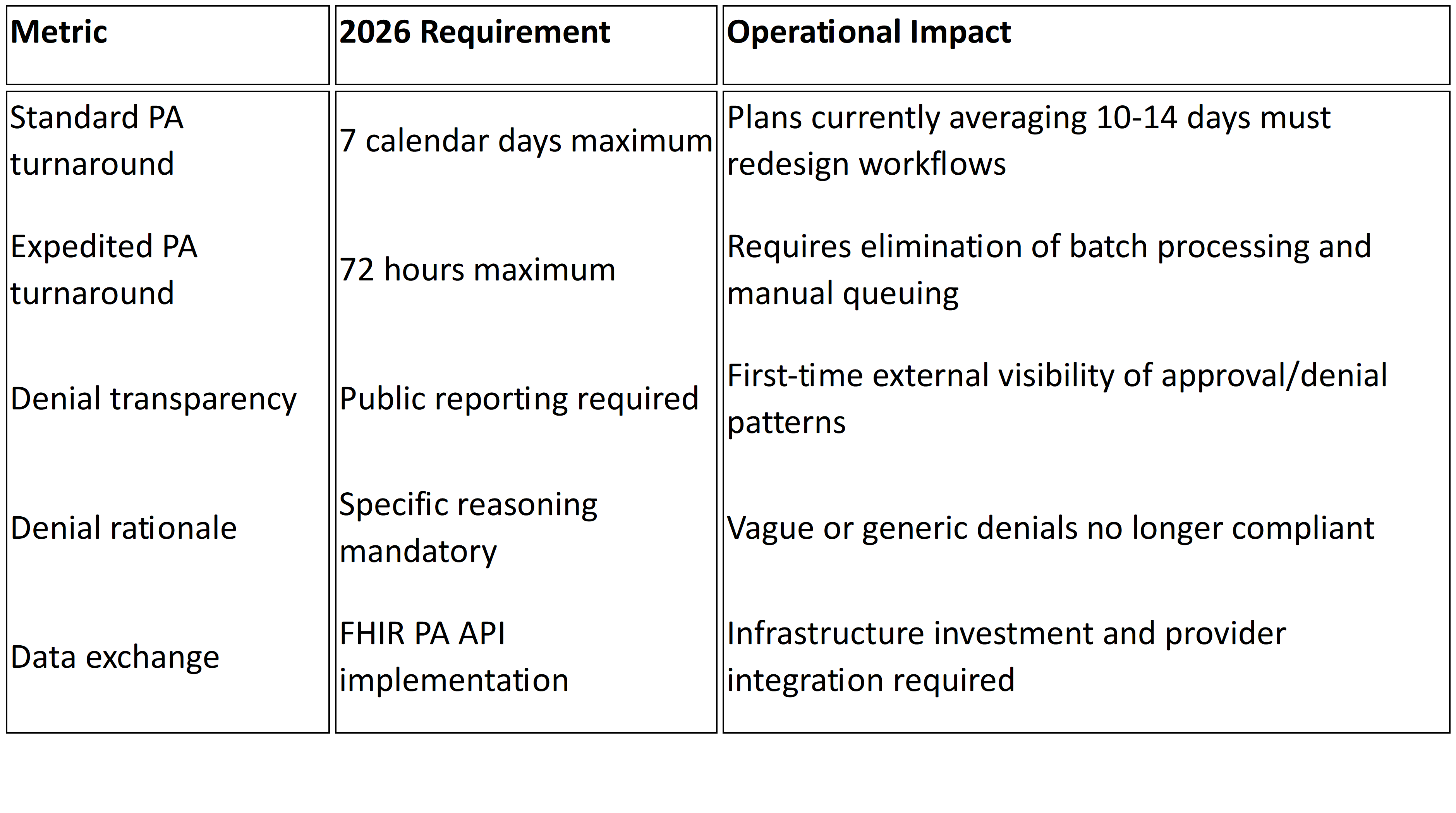
These aren't tweaks to existing workflows. They introduce enforceable timelines, public transparency, and standardized data exchange that most legacy UM environments were never built to support.
What This Exposes Inside Most Plans
The 2026 requirements don't create new operational weaknesses. They expose existing ones.
You Can't Hire Your Way to 72-Hour Compliance
If your prior authorization process depends on manual triage, inconsistent intake validation, or batch review cycles, meeting 72-hourand seven-day mandates becomes structurally challenging. Missed SLAs are no longer internal performance issues. They become regulatory violations.
The constraint is workflow design, not headcount. Adding clinical reviewers may temporarily reduce queue depth, but it doesn't eliminate intake latency, fragmented decision logic, or rework loops that consume days before cases reach clinical evaluation.
Your Denial Rates Are About to Become Public
For the first time, denial rates and processing times will be publicly reported beginning March 31, 2026.2
Plans with high denial rates, particularly those with elevated appeal overturn percentages, will face scrutiny from regulators, providers, and beneficiaries. Appeal overturn rates that were previously internal quality metrics become public signals about determination consistency.
Denials frequently reversed on appeal start looking less like utilization management discipline and more like systematic dysfunction.
Unstructured Intake Creates SLA Risk
Any workflow relying on fax, email attachments, or unstructured documentation creates intake uncertainty. Under 2026 mandates, that uncertainty translates directly into SLA exposure. What was operational inconvenience becomes regulatory vulnerability.
When requests arrive incomplete or in unstructured formats, the clock has already started but clinical review cannot. Days get consumed in follow-up and clarification before actual determination work begins.
Policy Fragmentation Becomes Audit Risk
Medical policies in PDFs. Coverage criteria configured separately in UM systems. Benefit rules embedded in claims engines.
When these layers diverge, denial rationale becomes inconsistent. Inconsistent rationale fuels appeals. Appeal patterns become public metrics tracked by CMS and visible to your provider network.
The 2026 rule requires "a specific reason for adenial"1 in a manner that allows providers to understand what additional information or clinical criteria would result in approval. Fragmented policy governance makes this level of specificity difficult to maintain consistently across thousands of determinations.
API Implementation Without Operational Alignment Fails
FHIR-based Prior Authorization APIs are mandated under the final rule1, but successful implementation requires more than technical connectivity.
These APIs demand structured, standardized data; clear mapping of coverage rules; real-time status tracking; and determination traceability. Treating API implementation as a technical bolt-on without aligning internal policy logic and workflow orchestration creates compliance on paper but operational brittleness in practice.
Reporting Infrastructure Will Strain Multiple Teams
Public reporting requires consolidated, accurate, reconcilable data. The rule requires payers to publicly report metrics including prior authorization decisions, denial reasons, and turnaround times2.
Most plans currently track these metrics across multiple systems: intake portals, UM platforms, claims engines. Without centralized reporting architecture, compliance becomes a manual reconciliation exercise rather than an automated output.
What Forward-Thinking Plans Are Doing Differently
The plans that will meet and leverage the 2026 expectations approach the problem differently.
They Treat Prior Authorization as a System, Not a Function
Rather than thinking in terms of "PA teams" or "PA tech stacks," they define a unified decision pipeline: intake →policy → decision → evidence → reporting. Every component must be architected for speed, traceability, and defensibility.
They Engineer Intake for Decision Readiness
Systems that treat intake as a validation and structuring event, not just data capture, dramatically reduce downstream review time. When requests arrive complete and structured, decisions get smarter and faster.
If a significant portion of requests require follow-up for missing clinical documentation, days burn before clinical review even starts. Fixing intake fixes throughput.
They Govern Policy and Logic Centrally
If policy resides in PDFs, disparate tools, and tribal knowledge, automation fails. Aligning policy logic, configuration, and deployment is the prerequisite for defensible, explainable decisions that meet CMS transparency expectations.
Centralized policy governance ensures reviewers apply consistent standards across all determinations, directly impacting appeal rates and public reporting metrics.
They Accelerate FHIR API Adoption Strategically
Forward-leaning plans are adopting FHIR Prior Authorization APIs now, enabling electronic request and response, reducing provider friction, and establishing a foundation for real-time decisioning rather than batch processing.
This isn't just compliance theater. It's infrastructure for the next decade of utilization management.
The 2026 Budget Reality
Most organizations' instinctive reaction to tighter SLAs is staffing expansion. Consider what that investment looks like:
Clinical hiring: Expanding nurse reviewer teams to handle faster turnaround requirements
Reporting resources: Staff to reconcile metrics across systems for public reporting compliance
API implementation: Technical infrastructure for FHIRPA API deployment and provider integration
Policy governance: Often unfunded, leading to continued fragmentation and appeal exposure
The alternative is investing in redesigning the decision pipeline itself. Structured intake, centralized policy logic, and automated workflow orchestration reduce review burden while improving consistency. The ROI isn't just compliance. It's operational leverage.
What This Means for Your 2026 Planning
If you're treating this as a compliance checklist, you're already behind. This is a fundamental redesign of how utilization management operates.
By Q2 2025: Audit your current PA workflow end-to-end. Identify where time gets consumed: intake validation, clinical review queues, policy lookup, documentation rework, peer-to-peer scheduling. Measure your actual turnaround distribution, not averages.
By Q3 2025: Centralize policy governance. Map coverage criteria to decision logic. Ensure clinical reviewers are applying consistent standards that can withstand public scrutiny and audit review.
By Q4 2025: Implement structured intake that validates completeness before requests enter clinical queues. Stand up reporting infrastructure that consolidates metrics in real time.
By Q1 2026: Conduct dry runs of public reporting. Simulate 72-hour expedited workflows under peak volume. Validate FHIR API functionality with key provider groups.
The plans that redesign now won't just comply. They'll operate with structural advantage.
How Mizzeto Helps Plans Redesign for 2026
We built Smart Auth after years of working inside health plan operations, seeing firsthand where prior authorization workflows breakdown. It's designed to make prior authorization decision-ready from intake through policy application and final determination.
Smart Auth structures data at intake, aligns policy logic centrally, and supports the traceability required for timely decisions and transparent reporting. It enables defensible, explainable determinations at the speed CMS expects without requiring massive clinical hiring or fragmented point solutions.
In 2026, prior authorization performance won't be judged internally. It will be measured, reported, and compared publicly. The question isn't whether to redesign. It's whether you start now or spend 2026 firefighting compliance gaps while your metrics become part of the public record.
Footnotes
- CMS Interoperability and Prior Authorization Final Rule (CMS-0057-F). Available at: https://www.cms.gov/newsroom/fact-sheets/cms-interoperability-and-prior-authorization-final-rule-cms-0057-f ↩ ↩2 ↩3
- CMS Interoperability and Prior Authorization Final Rule Informational Session, January 17, 2024. Available at: https://www.cms.gov/files/document/cms-interoperability-and-prior-authorization-final-rule-informational-session-january-17-2024.pdf ↩ ↩2 ↩3






























